In thermodynamics, heat engine refers to a system capable of converting the heat input to the system into mechanical work.
eg.: Steam engine.
In thermodynamic analysis, while solving problems a typical heat engine is represented as follow.
Here the heat engine acts between source and sink.Source will be at high temperature
eg.: Steam engine.
In thermodynamic analysis, while solving problems a typical heat engine is represented as follow.
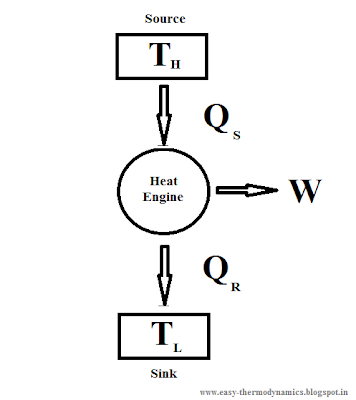 | |
| Representation of heat engine. |



